4.1 Transport in Peat
Similar to water flow, the size, shape, and connectivity of the pore network governs the advective transport of solutes in peat. A critical property of the peat pore network that influences both water and solute flow is its dual porosity structure discussed in Section 3, Peat: A Porous Medium. In peatlands, the distribution of pores from a predominance of macropores in near-surface peat to more numerous but smaller diameter pores at depth results in rapid shifts of transport rates vertically and horizontally. The increase in the abundance of small pores with depth also coincides with an increase of immobile porosity, 𝜙im, that does not contribute to advective flow. Solutes transfer to the immobile porosity from the mobile porosity via diffusion, which is driven by chemical gradients. Solutes that migrate into immobile porosity are abstracted from the solute flowing in the mobile porosity (Figure 17) with the net effect of retarding migration of the solute plume (Hoag and Price, 1997).
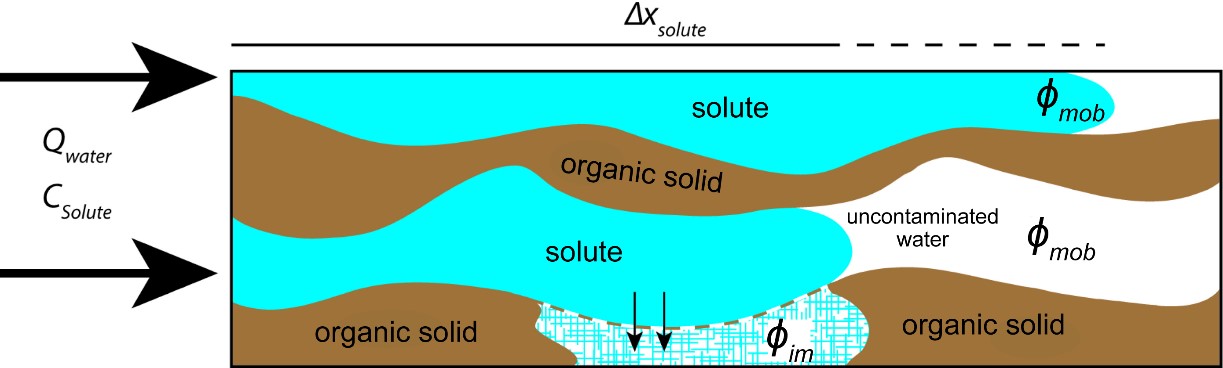
Figure 17 – Two pores within a peat without (top) and with (bottom) an immobile porosity. Blue represents pore water with dissolved solute, white represents pore water with zero concentration of solute, and brown represents organic matter. Both pores have the same input concentration (Csolute). The mobile porosity and its cross-sectional area are identical in both pores, and water flows through it at the same volumetric discharge rate (Qwater). The plume is shorter in the bottom pore as indicated by the solid portion of the black Δxsolute line above the figure because some of the solute mass that would otherwise be transported along with Qwater diffuses into the immobile porosity zone.
The parent material (e.g., Sphagnum moss, sedges, wood) has a large influence on the pore size distribution and the partitioning between the mobile and immobile porosity, thus plays an important role in solute transport. Estimation of mobile porosity and its influence on solute transport is explored in more depth in Box 5. However, most of our understanding of solute transport in peat and peatlands has been determined in the upper 1 m of peat from Sphagnum-dominated peats from bogs or poor fens. McCarter and others (2020) and Rezanezhad and others (2016) provide good discussions on solute transport in peat.
The majority of solute transport in peatlands is through highly connected, near surface, large diameter pores (pore diameters > 70 μm), primarily consisting of macropores (pore diameters > 250 μm). Solute transport through these pores is predominantly via advective flow. The high degree of connectivity, coupled with the predominance of large pores, results in low dispersivities on the order of ~10-1 — 100 cm (Hoag and Price 1997; McCarter et al., 2019). Dispersivity is a media and scale dependent property that represents both diffusion and variations in advection that are not accounted for in the flow portion of a solution in order to represent spread of a solute. As peat undergoes decomposition and compaction, such as with increasing depth below surface, dispersivity increases (Kleimeier et al., 2017).
Similar to water flow properties, Sphagnum and sedge peat differ in solute transport properties. In Sphagnum peat, the dispersivity and tortuosity is lower than that of sedge peat at an equivalent depth below the peat surface. This is due to the differences in pore structure, thus water flow, discussed previously. As such, the often systematic, small scale distribution of macropores and micropores, and the complexity of the pore network therein, is expressed at larger spatial scale, creating a clear region of enhanced solute transport. These regions of enhanced transport can be both vertically and/or laterally distributed as discussed in Section 3.2, Unsaturated Zone Properties and Processes.
The transfer of solutes within and between the mobile and immobile pore spaces is governed by the effective diffusion coefficient, which is both solute (the free water diffusion coefficient) and media specific (pore throat diameters). The free water diffusion coefficient is related to the ion size and the viscosity of the fluid—in the case of most peatlands, “fresh” water. The pore throat diameter is the size of the narrow portion of the opening between pores.
Although transfer of solute from mobile to immobile porosity zones is influenced by pore throat size, changes to the peat properties (increasing degree of decomposition) in the upper 50 cm of Sphagnum peat were not sufficient to decrease diffusion into the immobile porosity (McCarter et al., 2019). This is likely not the case in deeper, more decomposed peat, but there has yet to be measurements of this in deeper peats.
Although diffusion into the immobile porosity has been well established in peat, the mass transfer coefficients that are used to mathematically describe the process can be sufficiently high that solutes in the mobile porosity are essentially transferred to the immobile porosity instantaneously (McCarter et al., 2019). Under these circumstances, the peat can be simulated as a single porosity media with porosity equal to the mobile porosity (Simhayov et al., 2018). Currently, it is not known what specific discharge is low enough such that mass transfer rates between mobile and immobile pores are sufficiently high relative to the flow rate that dual porosity effects can be ignored (hence represented with a single porosity model). However, Kleimeier and others (2017) show that dual porosity effects were present at Darcy fluxes as low as 1.4 x 10-5 cm/s.
When the effective diffusion of solute in peat is low, the presence of an immobile porosity can lead to an elongated flushing of solutes, as the slower diffusive flux from the immobile porosity slowly transfers solutes to the mobile pore space (Hoag and Price 1997). This process can extend the period of contamination if a solute enters a peatland.
In peat, solutes are generally reactive due to the high organic matter content. Organic matter, whether considered peat or organic soil, removes cations from the pore water through adsorption. In peat, cation exchange capacities often exceed 100 centimoles per kilogram (cmol kg-1) and can be much higher than in clays and clay loams which are typically 30—50 cmol kg-1 and in sands are typically 3—5 cmol kg-1 (Kyzoil, 2002; Rippy and Nelson, 2007). This results in many cations being essentially immobile within all but the near-surface and high hydraulic conductivity peats where soil water residence times are low.
The mechanisms that govern cation adsorption are complex in peat. In Sphagnum mosses, cation adsorption is thought to occur in two different regions: the pore space and inter-cellular spaces. In soils that are not composed of living and dead plant cells, adsorption primarily occurs on the interface between the pore water and solid phase. However, the presence of plant cells creates a secondary region for adsorption to occur. In the leaves of Sphagnum, ions are transferred across the Sphagnum leaf’s cell membranes due to ionic gradients between the inter-cellular water and the pore water. Once within the inter-cellular space, cations adsorb to the cell wall. This allows for cations to affix throughout the leaf cell walls, not just the pore surface (Clymo, 1963; Richter and Dainty, 1989). Conversely, on the branches and stems of Sphagnum, direct ion exchange with the tissue surface is the dominant cation adsorption mechanism, which lowers the apparent adsorption capacity due to a decrease in available surface area.
The ability for cation adsorption depends on the physical location of the adsorption binding site and the highly variable chemical composition of the organic matter, thus the complexity of adsorption processes in peat. Most cations will undergo direct ion exchange with peat based on the peat’s overall negative surface charge at relevant pH from 4 to ~7 (McCarter et al., 2020). However, the presence of carboxyl groups and/or reduced sulfur groups (among others) in peat creates a large heterogeneity in adsorption potential depending on the specific geochemistry of a given cation. These complexes do not readily desorb, leading to relatively stable long-term removal of metals—and most cations—from peatland pore waters (Pratte et al., 2018). The heterogeneity in adsorption processes leads to differential transport rates of cations based on their specific chemistry, organic matter composition of the peat, and prevailing geochemical conditions, thus predicting contaminant transport at large spatial scales in peatlands can be difficult as discussed in Section 4.2, Transport in Peatlands.
Transport processes in peat and peatlands are governed by the prevailing geochemical conditions of both pore water and the peat due to the strong control pore structure has on solute transport. The decomposition of organic matter, in this case peat, controls the specific pore structures and, in many cases, the layering of peat within a peatland. These decomposition processes are governed by the soil moisture content, delivery of microbiologically available nutrients (e.g., O2, NO3–, SO42-), labile carbon to act as an electron acceptor, and the timely removal of decomposition end-products (Bauer et al., 2007). It is the balance, or imbalance, of these processes that allows for the accumulation of organic matter in peatlands and gives peat its unique structure. McCarter and others (2020) provide a more detailed overview of these processes and their interaction with hydrological and solute transport processes in peat.
