1 Introduction
Stable isotope hydrology is the study of water using the stable isotopes of the hydrogen and oxygen making up the water molecule. Many other stable isotopes can also be used to understand water, but are not part of the actual water molecule and are found within the dissolved matter (carbonate, nitrate, sulphate among others) in water.
As explained later in this book, the abundances of the different isotopes of hydrogen and oxygen vary as water moves through the water cycle. These variations are caused by reactions and transitions (e.g., evaporation and condensation). By measuring these variations, we are able to say something about the history of the water before it arrived at the point where we sampled it (Figure 1).

Figure 1 – The author collecting samples of snow for stable isotope analysis for his PhD, from Waaihoek Peak in the Hex River Mountains, Western Cape, South Africa. The snow was cored to enable analysis of the layers of snow from consecutive weather events and from the melted/evaporated surface layer between snow storms.
Many other chemical and isotopic methods are available to help us understand water and the processes it undergoes. However, all of the methods other than stable isotopes of water make use of dissolved substances or isotopes that occur in minor to trace quantities. For example, Cl is typically in the range of 1 ppm to 10,000 ppm, NO3 is seldom above 100 ppm, Sr isotopes occur at ppb levels, and 14C or 222Rn (radioactive isotopes) occur at even smaller fractions of the water being sampled. These tracers can therefore more easily be affected by precipitation, adsorption, dissolution and other reactions than the H and O isotopes of water.
The stable isotopes of water constitute nearly 100 percent of the water molecule and therefore suffer far less from reactions that might disturb the isotope abundances. For example, weathering of minerals will release Mg or Sr and change the chemical or isotopic abundance of such tracers as groundwater moves through an aquifer. The release of oxygen into the groundwater in the form of HCO3, H4SiO4 or other species, although able to exchange oxygen with H2O, will do so in such minor quantities relative to the circulating groundwater, that the stable isotope abundances of the water will not be affected. This behavior, where the species of interest conserves its composition through space and time, is called conservative behavior.
The main exception to the conservative behavior of stable isotopes of groundwater is in geothermal environments, where high temperatures increase the rate of reaction between rock and water, shifting the isotope abundances, particularly oxygen, due to its dominance in almost every mineral. Another exception to conservative behavior is where dissolved gases, such as H2S or CO2 interact with H2O, either chemically or through isotopic exchange, and thereby change the isotope composition of the water. These environments aside, the other main way in which the stable isotopes in groundwater may change over time is due to mixing with other groundwaters, which may or may not have different isotope abundances.
In many groundwater systems though, neither geothermal or geochemical reactions nor mixing occur, and the stable isotope composition of groundwater remains fairly constant from recharge point to discharge location. In these instances, variations in the stable isotope composition of groundwater must be due to variations in the recharge, or input to the system. Therefore, in order to understand the hydrogeology, we look to both surface and atmospheric waters to find the source of stable isotope variations. This means that, as hydrogeologists, we need to consider the complete water cycle and not only the portion occurring below ground, in order to make full use of stable isotopes. This is why we talk about stable isotope hydrology and not stable isotope hydrogeology (Figure 2).
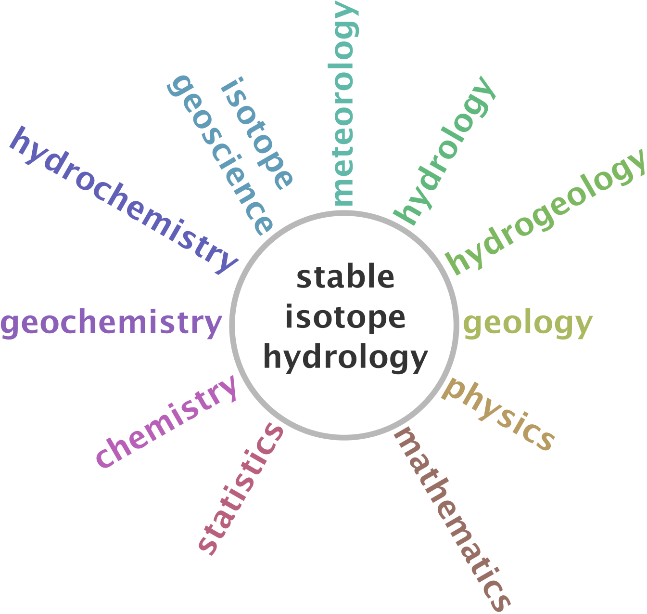
Figure 2 – Stable isotope hydrology interfaces with many other branches of earth science and the fundamental sciences, such as mathematics and chemistry.
The value of the preserved stable isotope compositions of groundwater is that they can then tell us something about the conditions at and prior to recharge. This is useful for understanding recharge and precipitation processes, including evaporation, intensity of precipitation, altitude of recharge and temperature of condensation. For example, if the groundwater is old, then it may provide insight into past climates and therefore forms one avenue in which scientists conduct paleoclimatic research.
This book outlines stable isotope hydrology at an introductory level as well as including some more advanced applications, aimed at students and professionals with a good understanding of hydrogeology, but little prior knowledge of isotopes. A good grounding in geology along with basic chemistry and physics is essential to being a good hydrogeologist. Knowledge of subjects such as meteorology, hydrology and geochemistry are also helpful at times. This book explains the occurrence of stable isotopes in water molecules and how they change abundance through the water cycle. Sections include measurement and reporting of data, calculation of meteoric water lines, the deuterium excess and other ratios, trends and patterns. The main body of the book explains how to use stable isotopes to perform various hydrogeological investigations and gives a series of case studies to show how these are done. This book also contains practical advice on water sampling for stable isotopes, some worked examples and exercises, as well as suggested reading for those wishing to delve deeper.
The overall intention of this book is that the reader will not only understand the theory, but also learn how to collect water samples, have them analyzed for stable isotope compositions, interpret the data and make conclusions useful for hydrogeologists or other earth scientists. This information, in turn, can be used by those managing natural resources, conducting site investigations or site rehabilitation and many other aspects of the ever-increasing field of environmental management.
