4.2 Equilibrium Fractionation
If chemical, physical or exchange reactions are allowed to run to completion, then there will be a fixed isotope difference between the source and receptor reservoirs, at a given temperature. Temperature plays an important role in fractionation. The higher the temperature, the less the degree of fractionation (Figure 7). Once equilibrium is reached, reactions will continue, but backward and forward reactions will occur at an equal rate, with no net effect on isotopic composition of either reservoir. For example, at a given temperature, there is a fixed difference between the 18O/16O ratio in H2O(l) and H2O(v) in equilibrium with each other. In this situation, the kinetic effects of reaction rate are not important, and it is the relative preference for a heavier or lighter isotope within a chemical bond that determines which isotopes are located where, with heavier isotopes favored in bond positions with higher strength. To continue the above example, the hydrogen bonds between water molecules are stronger between H218O–H216O than H216O–H216O, and so evaporation will preferentially select for H216O in the vapor mass, resulting in the H2O(v) having lower values for 18O/16O than the H2O(l).
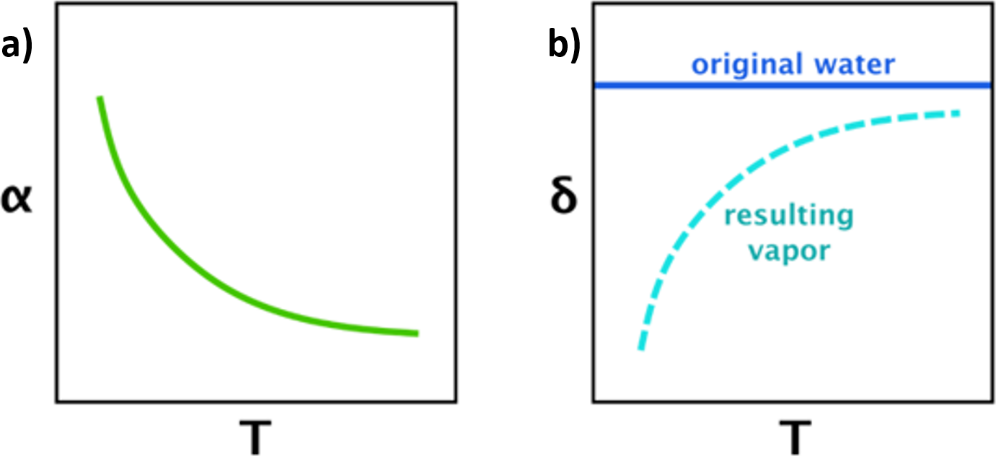
Figure 7 – Temperature dependence of fractionation. Graph a) shows the fractionation factor decreasing with rising temperature, and b) shows the difference in isotopic composition between a water source and its evaporated moisture becoming less at higher temperatures, assuming equilibrium conditions.
