5.1 The Global Meteoric Water Line
Stable isotope data from precipitation samples fall on a line known as a meteoric water line. This line is usually plotted with data from local, regional or global precipitation samples, but can include surface and groundwater samples. The Global Meteoric Water Line (GMWL) was first recognized by Craig (1961), based on fresh surface water samples from around the world (Figure 10), and is represented by Equation 8.
|
|
δ2H = 8δ18O + 10 |
|
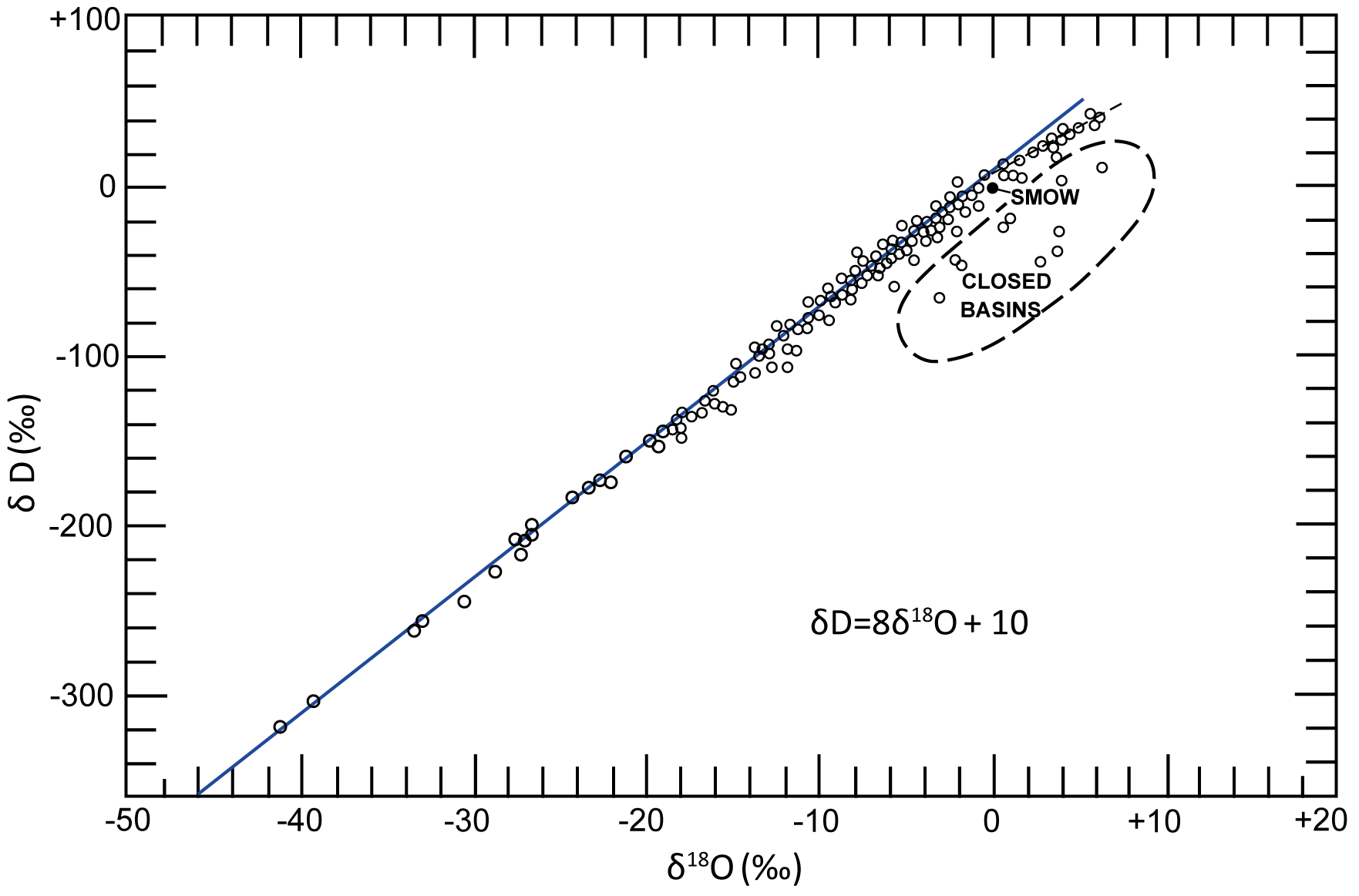
Figure 10 – The Global Meteoric Water Line (GMWL) as discovered by Craig (1961).
The spread of data along the GMWL is influenced by several meteorological processes or factors, such as humidity and temperature, but it is probably rainout of atmospheric moisture, as air masses move from the tropics to the poles, that accounts for the bulk of the variation in values (Rozanski et al., 1993; Yurtsever and Gat, 1981). As a moisture laden air mass moves from the tropics to the poles, moisture is removed by precipitation and the temperature tends to be lower. These lower temperatures not only encourage further condensation causing more precipitation, but enhance the removal of heavy isotopes by increasing the equilibrium isotope fractionation factors. As the process of rainout is governed by condensation, which is an equilibrium process, δ2H-δ18O co-vary, but with a factor of 8 difference. This factor of 8 difference is due to the ratio of the fractionation factors for H and O during rainout, which in turn is due to the difference in mass of 2H/1H being 8 times greater than the difference in mass of 18O/16O.
[latex]\frac{{ }^{18} O-{ }^{16} O}{{ }^{16} O}=\frac{2}{16}=\frac{1}{8} \text { and } \frac{{ }^2 H-{ }^1 H}{{ }^1 H}=\frac{2-1}{1}=1[/latex]
Thus, the variations in δ2H will be roughly 8 times those of δ18O. The difference in mass causes a difference in energy needed to break the bonds, and that is the ultimate cause of fractionation. However, temperature changes the 2H/1H and 18O/16O equilibrium fractionation factors differently, so the gradient of 8 steepens in colder regions and lessens in warmer regions (Clark, 2015). The value of 10 is the intercept, or the δ2H value when δ18O is 0.
The GMWL was updated by Rozanski and others (1993) and then again by Araguas-Araguas and others (2000) to δ2H = 7.96 δ18O + 8.86.
