6.6 Geothermal Waters
Heated groundwater can be divided into two types, although there is a continuum between the two. The term thermal (or hot) springs usually applies to groundwater that is noticeably warmer than the mean annual temperature of an area, so the temperature definition varies with the local climate. For temperate climates, 15 °C is a typical threshold between shallow unheated groundwater and geothermally heated water. The threshold is closer to 20 °C for Mediterranean climates, around 25 °C in the tropics, and up to 35 °C in hot deserts. The depth to which groundwater must circulate in order to be heated again varies from region to region, depending on the geothermal gradient, which is influenced by the age and thickness of the crust and the degree of volcanic activity in the area. However, there is usually a clear distinction between relatively low geothermal gradients of around 10 to 30 °C/km in tectonically quiet areas (most of the earth’s surface), versus the much higher geothermal gradients in volcanically active areas where boiling water and steam occur within tens to hundreds of meters of the surface. These latter areas are usually referred to as geothermal.
From an isotopic perspective, thermal springs are usually caused by deep circulation of meteoric water, usually at temperatures below those likely to cause isotope exchange with rocks. As such, studies evaluating recharge and flow paths, and other hydrogeologic issues, can be conducted using the stable isotopes, as they are conservative, for example as shown in Figure 26 (Chandrajith et al., 2012; Diamond and Harris, 2000; Durowoju et al., 2019). Most of the concepts in this book are applicable in these situations.
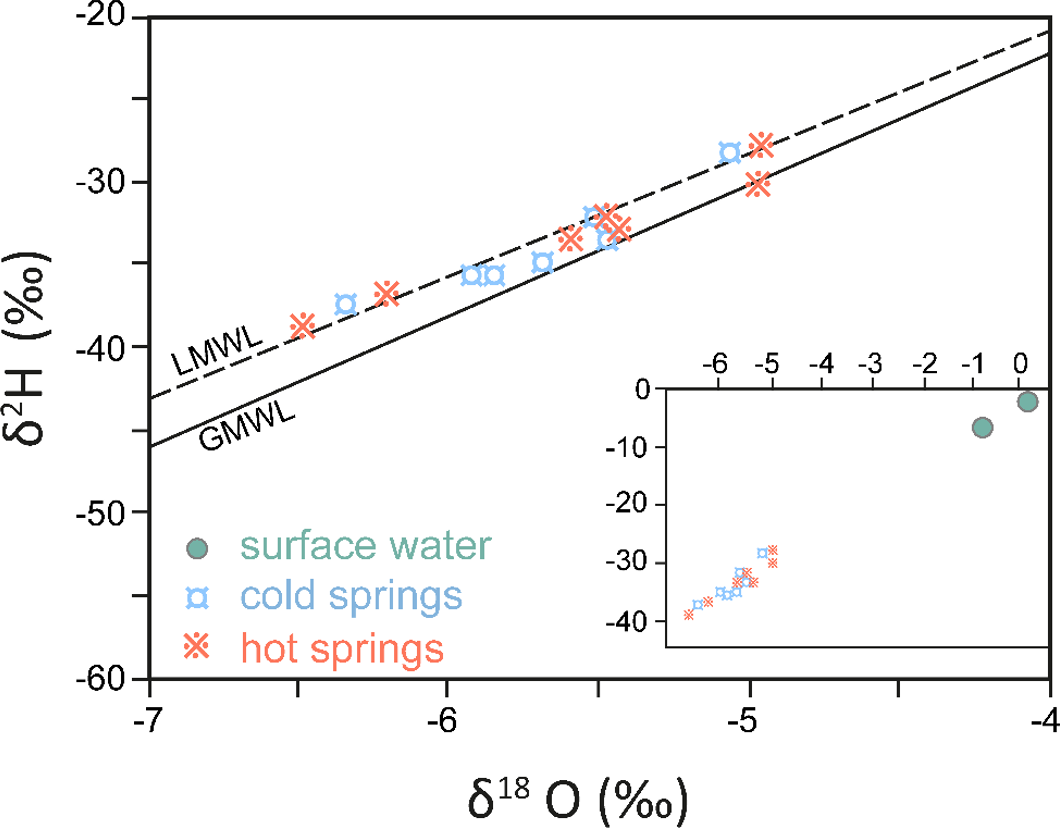
Figure 26 – Stable isotope data for cold and hot springs (< 62 °C) in eastern Sri Lanka. The overlap of isotope compositions for hot and cold springs is typical for lower temperature thermal springs. Surface water typically has heavier isotope composition of the groundwater as displayed in the inset (after Chandrajith et al., 2012).
In contrast, geothermal waters usually experienced isotope exchange with the host rocks resulting in the isotope composition changes (Lelli et al., 2021). Figure 27 shows how isotope exchange, caused by the high temperatures of geothermal fields (> 100 °C), with silicates, which generally have positive δ18O values, is the cause of variation in isotope composition. If mixing with juvenile (magmatic) water was the cause, the δD values of the geothermal water would have changed and the mixing lines from the various geothermal fields would converge on the juvenile water type.
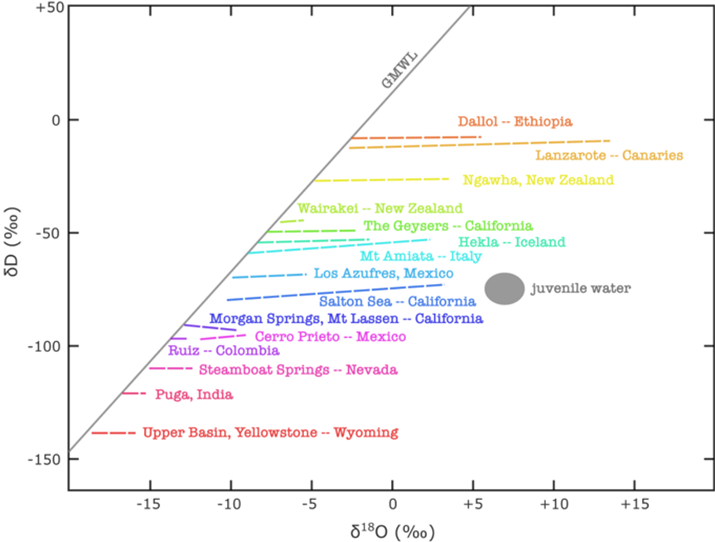
Figure 27 – Stable isotope compositions for water and steam at geothermal locations around the world, in comparison to the GMWL (Global Meteoric Water Line). Mixing with juvenile (magmatic) water is not the cause of variation from meteoric water, but rather the variation is caused by isotope exchange with silicates. This is revealed by major changes in oxygen isotopes, while changes in hydrogen are negligible because of its lesser abundance in minerals (after Panichi and Gonfiantini, 1981; D’Amore and Panichi, 1985; Stewart, 1985).
In the case of Wairakei and some of the other fields, the shift in δ18O is not large. This likely occurs because the rocks already exchanged oxygen with the groundwater over many years and now the system is close to the equilibrium fractionation value with the meteoric water, so further exchange is minor. Extending this concept, the factors responsible for determining the degree of isotope exchange between water and rock are: original isotopic composition of the rocks, original isotopic composition of the meteoric water, quantity of rocks, quantity of water, temperature, water circulation path, the water/rock contact-surface area, and time (Panichi and Gonfiantini, 1981; Daniele et al., 2020).
Groundwater temperatures in geothermal fields are very high, so it is expected that, given the chance, significant evaporation will occur. This evaporation can either be at the surface, or in the subsurface, and there are ways to model both the amount of evaporation and the process. These processes include single-stage or continuous steam separation, the former being when steam is released only once the groundwater-steam mixture arrives at surface, the latter being where steam is continuously removed from the water as it boils underground. Work has been done monitoring the changes in hydrochemistry and isotopes during exploitation of geothermal fields to provide estimates on the sustainability of the field (Prasetio et al., 2021).
Finally, stable isotopes of water and another compound, such as CH4 or CO2, can be used to estimate the temperature underground. For example, if the hydrogen isotope composition of water and methane is measured at surface, and these are assumed to be in equilibrium, then the temperature of reaction can be estimated from the known change in fractionation factor with temperature. Several assumptions need to be fulfilled, but the method can be made more robust by using other geothermometers, such as oxygen isotopes in water and sulphate (Panichi and Gonfiantini, 1981).
