3.1 Mass Spectrometry
Mass spectrometers were first developed in the early part of the 20th century, but the precision was only sufficient for application to natural stable isotope variations after World War II. Thus, was born the field of stable isotope hydrology (Gat, 1981; Sharp, 2007). Today there are many types of mass spectrometers, with specialized designs and features enabling a wide variety of analyses to be performed on samples as diverse as rocks, gases, proteins and teeth. The laboratory preparation procedures are equally diverse for all these substances. Hydrogen and oxygen isotopes of water are typically analyzed in gas-source, light-isotope ratio, mass spectrometers (IRMS) specially adapted for the stable and light isotopes, which include carbon, nitrogen and sulfur.
Mass spectrometers work in the following general way (Figure 5). First, the sample is introduced into the machine, either as a gas, or by vaporizing a liquid or solid sample. This gas or vapor is then ionized using a variety of techniques, such as chemical ionization, electrospray ionization or inductively coupled plasma. The ions are then accelerated and focused down the flight tube and through a strong magnet, where electrical and magnetic fields cause deflection of the ions. This deflection is dependent upon the charge of the ion, as well as its mass. For ions of the same charge, the heavier ions will deflect less, as they possess more momentum than the lighter ions. The last part of the process is the detector, containing collector cups, amplifiers and other electronics to record, process the signal, and output ratios of the various masses of ions being emitted from the source.
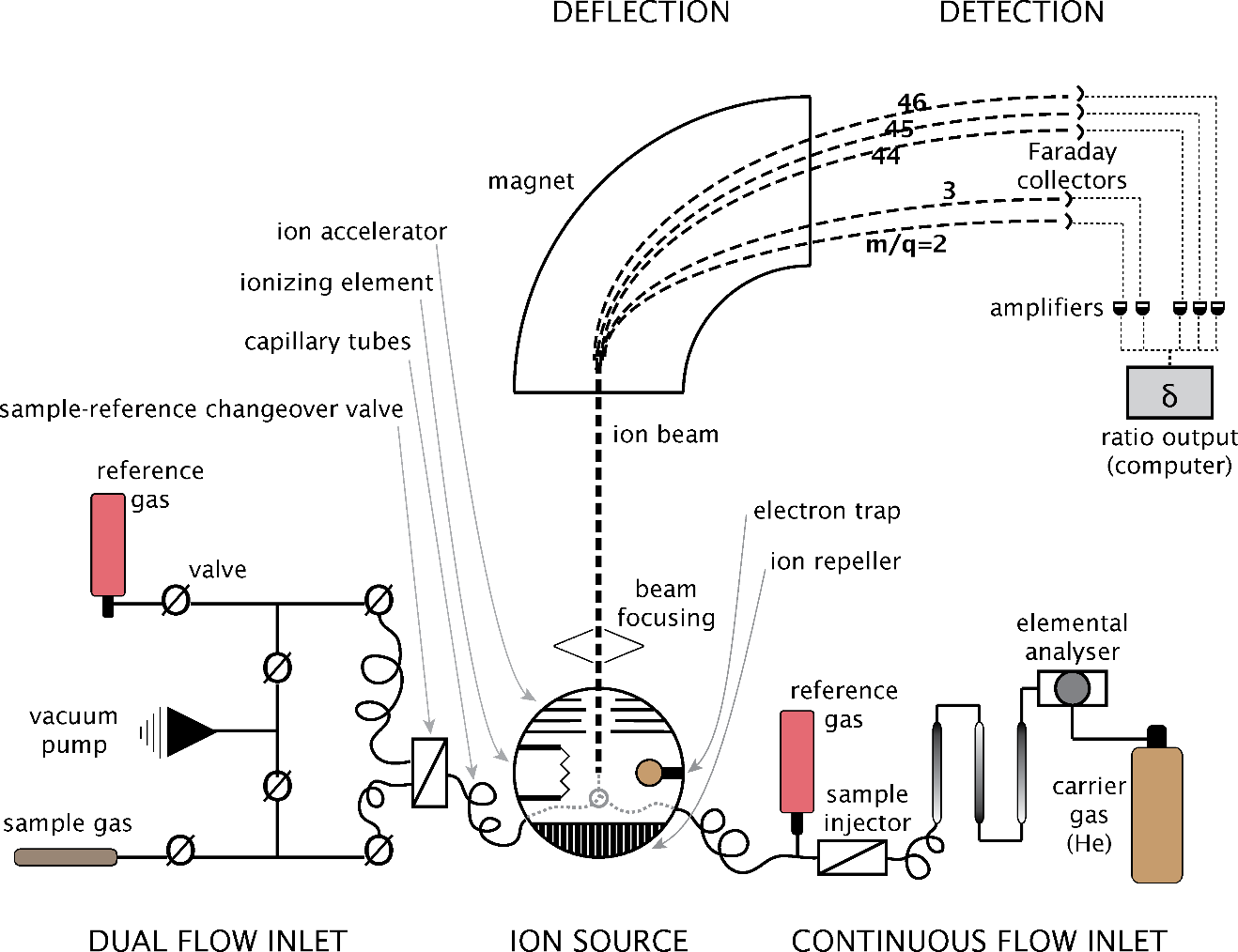
Figure 5 – Schematic diagram of a gas-source, light-isotope-ratio, mass spectrometer, including the two main types of sample injection setups, continuous-flow and dual-inlet. The m/q (mass/charge) ratios are illustrative of the paths taken by various ions. Ions with lower m/q ratios are deflected more by the magnet.
For water, the gases H2 (for δ2H) and CO2 (for δ18O) are used as the inputs to the mass spectrometer. Sample preparation procedures are outlined in Schimmelman and others (1993) and Socki and others (1992), although other methods are available.
IRMS makes use of either a dual-inlet system, or a continuous flow system. Water has generally been converted to gases, so the dual-inlet system is mostly used. For the dual-inlet system, the samples are alternated with the reference gases, providing a high level of precision. For the continuous flow system, a sample is analyzed only once and the precision is therefore not as good. Laboratory standards are inserted every ten to twenty samples, allowing correction of any drift in the reference gases. These laboratory standards are calibrated to the international standards, such as SMOW, allowing the researcher to present their data in an internationally recognizable form. Paul and others (2007) discuss normalization of the sample data to the standards.
