6.7 Paleowaters
Paleowaters in groundwater include any water that was recharged under a different climate from the present one or formed under different environmental conditions to those now found at the site. For hydrogeologists, who are more concerned with circulating groundwater, waters involved with the genesis of rocks and minerals are generally of less interest as they are not an active part of the hydrological cycle and therefore do not represent a sustainable water resource. As such, connate water, formation water, juvenile water, interstitial water and crystallization water are excluded from the usual definition of paleowater.
Paleowater groundwater resources are called fossil aquifers and are sometimes used for water supply. An example is the Nubian Aquifer System of the eastern Sahara, shown in Figure 28. Roughly speaking, actively circulating groundwater is likely to be less than 10,000 years old, paleo groundwaters are in the range of tens to hundreds of thousands of years old, and connate, crystallization, formation, interstitial and juvenile waters are more likely to be millions to billions of years old.
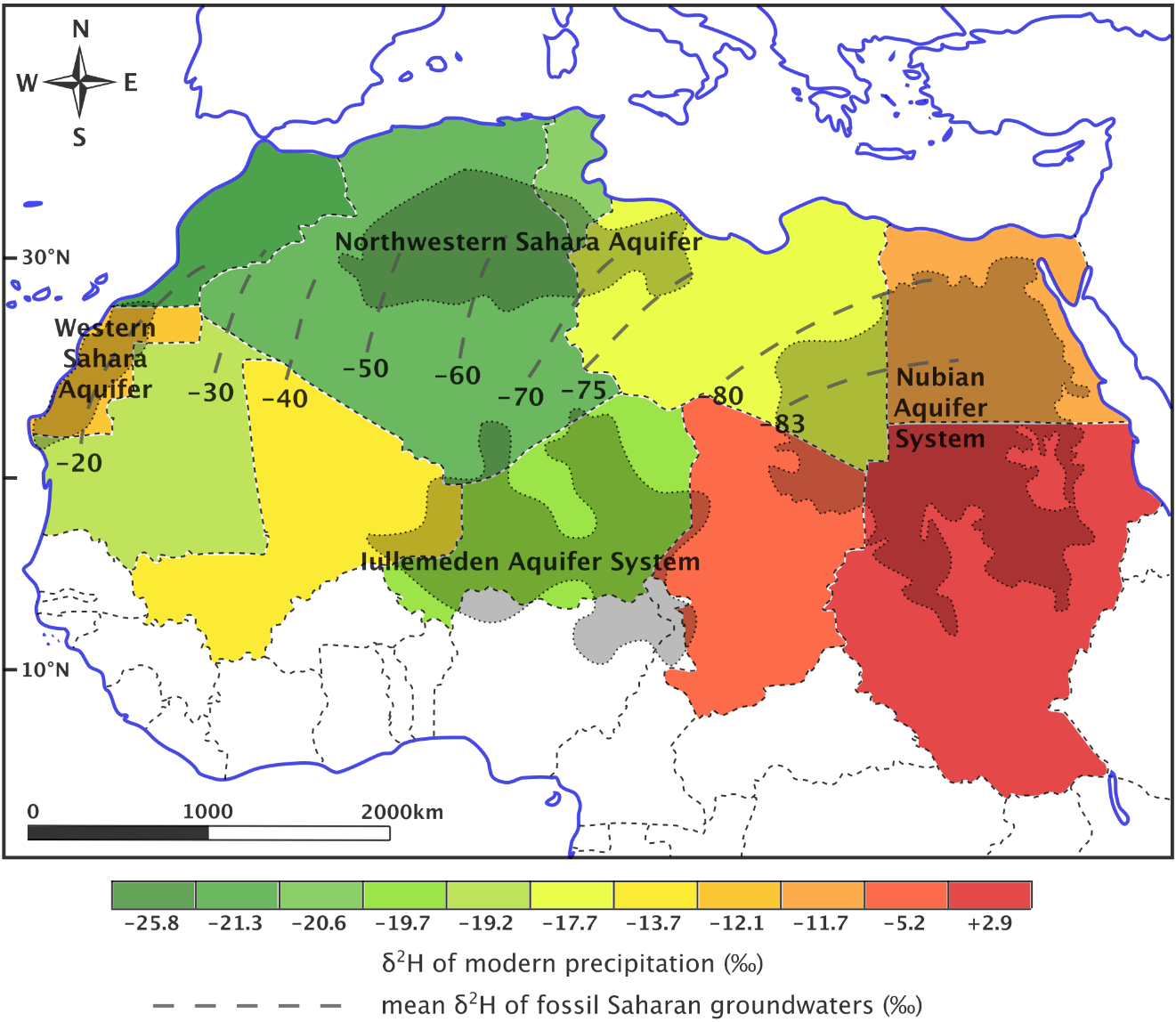
Figure 28 – Contrast in isotope patterns for modern precipitation (shown by color-coded country outlines) versus paleowaters (shown by dashed contour lines) across the Sahara Desert. The west to east decrease in δ2H for paleowaters was used as evidence for an shift in westerly rain systems toward the equator during the last ice age (after Abouelmagd et al., 2012).
A common requirement for working on paleowaters is the ability to date the groundwater age. Tritium, although commonly used in young groundwater, is of no use in paleowaters as its half-life is too short (12.3 years). Carbon-14 (half-life 5730 years) is the most widely used dating method, but, as explained in another Groundwater Project book, Introduction to Isotopes and Environmental Tracers as Indicators of Groundwater Flow (Cook, 2020), this is far from simple. The quantity of 14C in the groundwater is given as pmc (percent modern carbon), from which the age can be calculated, however, there are complications which can cause this calculation give the wrong age. On the one hand the 14C can be removed by preferential precipitation or diluted by contributions of inorganic carbon dissolving out of the aquifer matrix, and on the other hand modern carbon can enter the system and mix with the old groundwater or contaminate the water during sampling. In general, therefore, although the analytical precision can theoretically date materials to 60,000 years, the uncertainties in the groundwater environment reduce this method’s age maximum to around 30,000 years (Clark and Fritz, 1997; Fontes, 1981).
Other dating methods for paleowaters can also be used, for example 36Cl with a half-life of 301,000 years. This and other methods have advantages and disadvantages and have certain age ranges over which they may give reliable ages. Groundwater dating is a vast field and the reader is encouraged to consult other books on these topics, if they want to know more about it (Clark, 2015; Cook, 2020).
Although accurate groundwater dating is the surest way to know one is dealing with paleowater, discovering groundwater with a stable isotope composition different from groundwater known to be young, often alerts one to the presence of paleowater (Van Gelden et al., 2014). In most cases, the paleowater is in deeper, confined aquifers with little connection to unconfined aquifers with modern recharge (McIntosh et al., 2006), but there are instances where the geometry of aquifers and flow regime can result in the paleowater occurring at shallow depths (Malov and Tokarev, 2019).
Identifying the presence of paleowater from a difference in stable isotope composition with the young groundwater of the region is useful. One of its uses is to understand the sustainability of groundwater, because use of paleowater is generally assumed to be non-sustainable. This is because paleowaters were either recharged during a wetter climate and are not being recharged at all in the current climate, or are recharged very slowly, hence their great age, and therefore only sustainable at very low abstraction rates. Al-Charideh and Kattaa (2016) investigated groundwater in the regional, deep Cretaceous aquifer across western Syria, and were able to classify groundwater as renewable or semi-renewable when the stable isotope composition was similar to modern precipitation (δ18O of -7.0 to -7.2‰), or as non-renewable when the stable isotope composition was significantly more negative, suggesting recharge during a previous climate (δ18O of -8.0‰). Additionally, 14C values for the latter groundwater were < 15 pmc, confirming this as older groundwater.
A study by Smith and others (2002) in the Great Basin region (centered on Nevada, USA) identified locations where δ2H of groundwater was more than 10‰ lighter than winter precipitation. They proceeded to rule out sites adjacent to mountains, where the altitude effect could be the cause of the isotopically lighter groundwater values, and then classified areas as having possible paleowater (10-19‰ lighter) and probable paleowater (>20‰ lighter), recharged during the Pleistocene.
In previously glaciated parts of the world where meltwater from receding glaciers recharged groundwater as the previous glacial period ended, differences in paleowater and modern groundwater isotope compositions can be large. However, in some cases the paleowater in far northern latitudes are not from glacial meltwater and can therefore have differences to modern waters that are similar to those in mid-latitudes or tropical areas. For example, in Germany, Van Gelden and others (2014) noted the similarity of modern precipitation (-9.8‰ and -66‰) and the shallow, unconfined aquifer (-9.6 to -8.9‰ and -68 to -65‰) compared to the confined Benkersandstein (-10.6‰ and -74‰) giving roughly a 1‰ and 10‰ difference in δ18O and δ2H respectively, between modern (shallow groundwater or precipitation) and paleowater. In contrast, in the Great Lakes region of North America, McIntosh and others (2006) found current precipitation and surficial aquifers to have δ18O of -7 to -4.5‰ in northern Indiana and Ohio, and -11 to -8‰ in northern Michigan, whereas the Silurian-Devonian carbonate aquifers were in the -13 to -15‰ range, exhibiting a difference of 5-10‰ in δ18O, as shown in Figure 29.
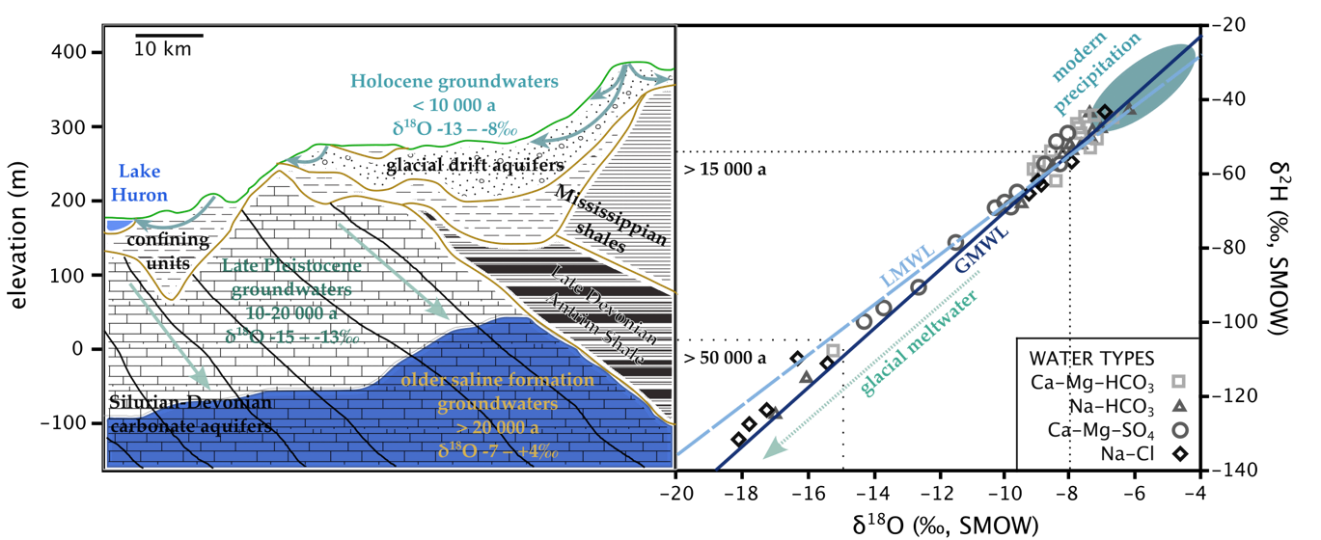
Figure 29 – Groundwater regime and stable isotope systematics for northern Ohio and Indiana states in the USA, south of the Great Lakes. Various groundwater samples are plotted on the δ2H – δ18O graph according to their dominant dissolved ions. The estimated isotope composition for glacial meltwater ranges from -25 to -11‰ for δ18O. Stable isotope ranges for paleoprecipitation are shown as dotted boxes with associated ages. The CaMgHCO3 waters are associated with the shallow, unconfined glacial drift aquifers and tend to have stable isotope values somewhat more negative than modern precipitation, but plotting close to the LWML and GMWL. The other water types are found in confined aquifers, at depth, and their chemistry is influenced by rock dissolution and mixing with formation brines. The existence of isotopically very negative groundwater at shallow depths in the Silurian-Devonian carbonate aquifer suggests this groundwater was recharged by melting glaciers at the end of the last ice age and is largely isolated from modern recharge by aquitards (after McIntosh et al., 2006).
In much of the southern interior of Canada and parts of the northern plains of the USA, there is abundant paleowater within the Quaternary deposits overlying bedrock. As the Pleistocene glaciers were melting (about 10,000 to 20,000 years before present), run-off from these glaciers formed lakes with deposits rich in silt and clay across broad plains. These deposits have exceptionally low permeability (with thicknesses from 5 to 40 m) and function as aquitards with the water table positioned near the ground surface. Studies of these aquitards show that where the thickness is greater than ~20 m, the groundwater is comprised mostly or entirely of water derived from the Pleistocene glaciers (Figure 29). This conclusion is based on stable isotopes indicated by cold weather isotopic signatures.
One of the most extensive deposits of these glacial lake sediments occurs in Manitoba, Canada, and extends southward into North Dakota, USA. A uniform oxygen isotope value of –25‰ was obtained at depths of 20 to 30 m in a thick deposit of clay found in the southernmost part of the glacial Lake Agassiz plain (Remenda et al., 1994). The lake occupied parts of North Dakota and southern Manitoba at the end of the last glacial maximum and received water from the ice margin and the interior plains region of Canada. The value of –25‰ is characteristic of meltwater impounded in the southern basin of Lake Agassiz. This value corresponds to an estimated mean air temperature of –16 °C, compared with the modern mean air temperature of 0 °C for this area.
Groundwater from thick, late Pleistocene-age clay deposits elsewhere in Canada shows the same uniform oxygen isotope value at similar depths, including a glacial till in southern Saskatchewan and a glaciolacustrine deposit in northern Ontario. These aquitards are at about 50 °N latitude, span a distance of 2000 kilometers, and like the Lake Agassiz sites, have a groundwater velocity of less than a few millimeters per year. Hence, there is a substantial volume of this paleowater in the southern interior regions of Canada, but almost all of this water occurs in aquitards where flow rates are minimal, rather than in aquifers. This is because water of glacial origin, that initially filled the aquifers, has flushed out since the glaciers disappeared. An exception is the regional aquifer beneath the lake sediments in southwestern Ontario where the aquitard is an effective cap, impeding the flow of paleowater (Husain et al., 2004). The glacial age of these paleowater occurrences has been confirmed by carbon dating. However, given the characteristically negative stable isotope values for the paleowater in this region, the most cost-effective method for dating paleowater is through the use of stable isotopes.
The differences in stable isotope composition for modern water and paleowater may be known in a region. Sultan and others (2000) applied such regional knowledge to the unconsolidated aquifers close to the Nile River, south of Cairo, near Wadi El Tarfa. These aquifers had δ18O of -2 to +5.2‰ and δ2H of -10 to +34‰, which ruled out upwards leakage from the Nubian Aquifer, known to have mean values of -11‰ and -80‰, respectively (Figure 30). They concluded that recharge of the shallow aquifers was from flash flood events with rare, but appreciable recharge into coarse sediments and fractured rock underlying alluvial channels. As such, these aquifers can be sustainably used, if the frequency and magnitude of recharge from these events is known.
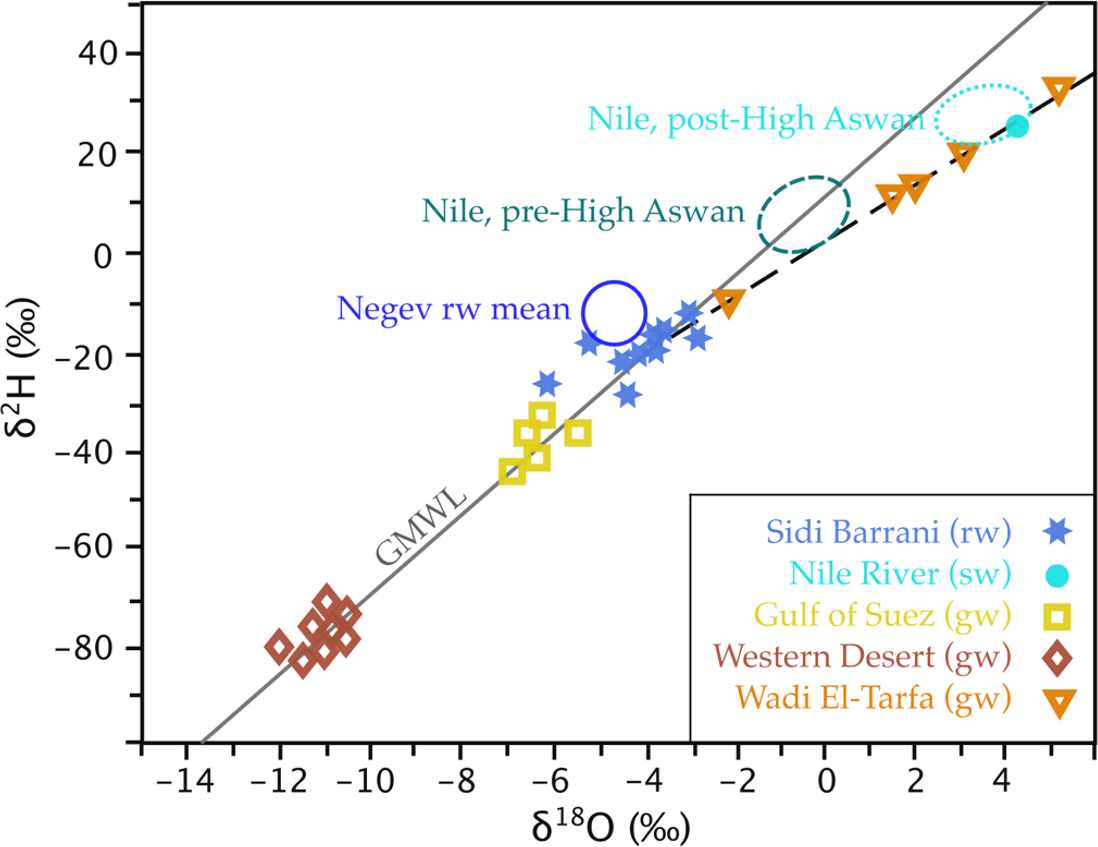
Figure 30 – Stable isotope analyses of various waters in Egypt and rain from the Negev Desert in Israel, with the GMWL and a trend line for Wadi El-Tarfa groundwater, in order to determine the likely source of Wadi El-Tarfa groundwater. Upwards leakage of paleowater from the Nubian Sandstone Aquifer of the Western Desert was ruled out due to its lighter isotope composition. The position of the Wadi El-Tarfa samples below the GMWL is suggestive of evaporation. The dashed trendline has a gradient of 5.7, indicating evaporation from an open water body, such as a temporary pond after heavy rain. The extrapolation of the trendline into the cluster of Sidi Barrani rain water suggests evaporated modern rainfall recharges the Wadi El-Tarfa groundwater, meaning abstraction is potentially sustainable at a rate similar to the recharge (after Sultan et al., 2000).
In nearby Libya, Al Faitouri and Sandford (2015) conducted a survey of three sandstone aquifers within the Nubian Aquifer System, to establish paleoclimatic and recharge dynamics. However, the stable isotope characteristics of these three aquifers can also be used to detect leakage or mixing of groundwater, by monitoring the pumped groundwater for changes in stable isotope composition over time. Initial results suggest that younger groundwater is being incorporated into the deeper aquifers as the potentiometric head of the deeper aquifers has been lowered and therefore flow induced from the shallower, younger groundwater.
Work on the Nubian Aquifer System, as well as other paleowaters in the Saharan region, has extended to paleoclimatic reconstruction. Using d-excess values calculated by Sonntag and others (1979) for 20,000-year old groundwater beneath the Sahara, Merlivat and Jouzel (1979) were able to, with the aid of an isotopic precipitation model, calculate the humidity under which evaporation took place over the ancient ocean, finding a result of 90 percent as compared to 80 percent for the current climate. Abouelmagd and others (2012) showed how the average modern precipitation can get isotopically heavier from west to east (W to E), but the paleowater gets isotopically lighter from W to E (Figure 28). However, some modern rain events show isotopic depletion from W to E, matching the fossil groundwater. These were produced with westerly winds and so it was inferred that the paleoclimatic recharge was due to the westerlies (mid-latitude cyclones or frontal depressions) shifting toward the equator during the previous ice age.
In contrast to the findings in the Sahara, Plummer and others (2012) found high D-excess values for paleowater in the vicinity of Chesapeake Bay, USA, indicating lower relative humidity during moisture formation (Figure 29). This is consistent with the colder temperatures and icy conditions expected over mid-latitudes during glacial periods. The higher humidity detected for paleowater recharge conditions over the Sahara and the lower humidity over the American mid-latitudes agree with the general model that current polar conditions (cold and dry) occupied mid-latitudes and current temperate conditions (wet and rainy) occupied low latitudes during the previous ice age.
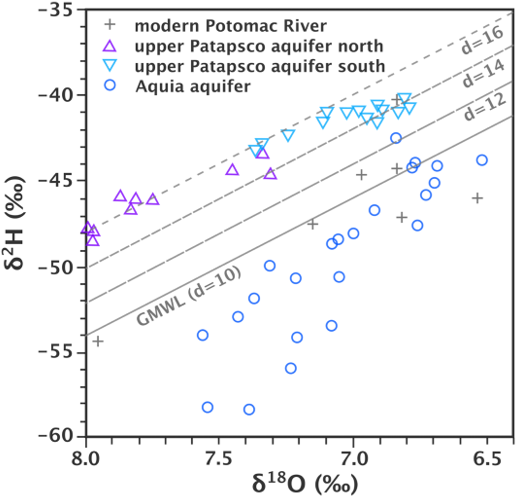
Figure 31 – Stable isotope values for groundwater from various aquifers in the Chesapeake Bay region. Distinct groups are apparent for the various aquifers, reflecting differences in climate, and particularly the d values, which is an indicator of the humidity level in the region that provided the moisture source. Higher d values indicate lower humidity in the moisture source region (after Plummer et al., 2012).
Stable isotopes of groundwater may also be used indirectly in paleoclimatic and other reconstructions. Andrews (2006) provides a review of the use of groundwater-fed riverine tufas (carbonate deposits) for these purposes. The method used the δ18O of the carbonates, corrected for fractionation at the temperature of precipitation from water, to determine the δ18O of the groundwater at recharge. High resolution sampling of tufas (sub annual scale) tends to provide more information on the temperature of precipitation (precipitation), whereas decadal scale sampling gives more information on the groundwater isotope composition, and is susceptible to the well-known isotope effects of continentality, amount and temperature. These allow some inference regarding the types of events responsible for groundwater recharge. For north-western Europe, tufas revealed centennial scale cooling prior to the end of the last ice age, reaching a minimum temperature around 8200 years before present. Tufas are therefore a good alternative or complement to paleoclimatic methods using speleothems.
Canavan and others (2014) performed analyses of the δ2H of volcanic glass in the central Andes. Primary volcanic glass has only 0.1-0.3 weight percent H2O, whereas after hydration (thousands of years) the glass contains about 5 weight percent H2O, and the hydration seals in the isotopic content of the hydration water with a layer of “silica gel”. This means the hydrogen is largely sourced from meteoric water and the original δ2H of hydrating water can be calculated after applying the fractionation factor for glass-H2O. With this paleowater δ2H signature, and applying known altitude isotope gradients, the elevation at the time of eruption (given that hydration is achieved shortly thereafter) can be calculated. This in turn can be used to infer uplift rates and continental geodynamics of a region.
It should be apparent that stable isotopes of paleowater, and associated materials (e.g., speleothems, tufas, volcanic glasses) are a mine of potential information. However, the corrections and complexities require attention, to avoid misinterpretation. Geological dating, in particular, is very useful in this area, but attention to fractionation, mixing, dilution and other factors is necessary. As is often the case, use of hydrochemistry or other isotope systems can assist in painting a fuller picture.
