6.2 The Deuterium Excess
Kinetic fractionation during evaporation from the ocean surface takes place because of diffusion of water vapor molecules from a saturated boundary layer at the sea surface and into the open atmosphere. The 1H1H16O isotopologue diffuses faster than all the others and so the vapor in the open atmosphere is depleted in the heavier isotopes. If the atmosphere was saturated, then isotope exchange would occur fully between the sea and water vapor in the air, resulting in isotopic equilibrium.
Importantly, there is a difference between the diffusion fractionation factors for 2H-1H and 18O-16O and so the relative depletion of 2H and of 18O is not the same. This difference between the rate of 2H depletion and 18O depletion changes with the degree of saturation, or relative humidity, h. In other words, at lower humidity, 2H is not affected by evaporation as much as 18O, which means vapor at lower humidity will be further from the GMWL. As h increases, more isotope exchange will occur and the fractionation the system will come closer to equilibrium (Clark and Fritz, 1997).
This means the position of a LMWL is partly a function of the relative humidity of the source region, where evaporation generated the vapor mass. Evaporation under low relative humidity will generate lines displaced further left of the GMWL. Figure 20 shows how evaporation from sea water under 85 percent relative humidity conditions and then condensation at equilibrium generates water with isotopic compositions that plot along the GMWL. At various values of relative humidity, vapor and the resulting water samples will be displaced from the GMWL to a greater or lesser degree and will have an intercept on the δ2H axis that is larger or smaller than 10. This intercept, known as the deuterium excess (D-excess, or d) of a water sample is calculated with Equation 13.
|
|
D-excess = δ2H – 8δ18O |
|
where:
|
δ2H and δ18O |
= |
values for the water sample |
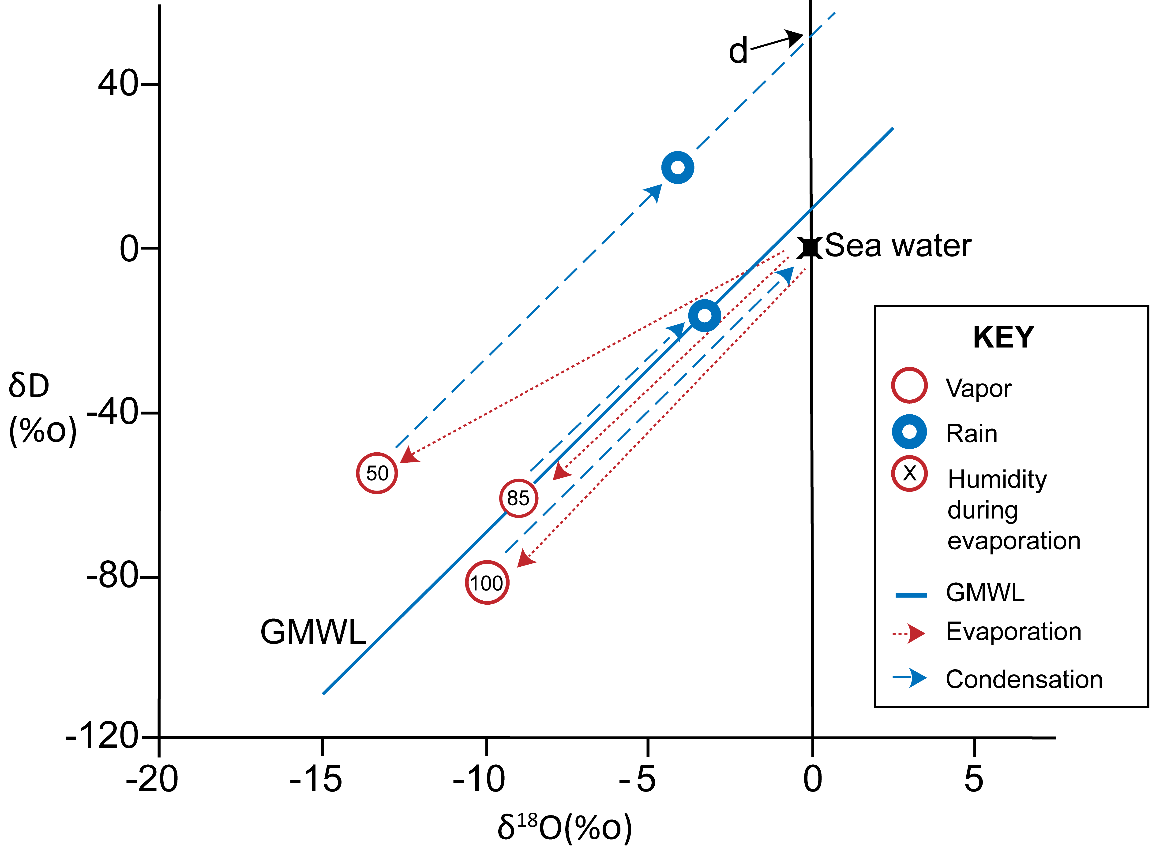
Figure 20 – Different relative humidity in source regions during evaporation (red dotted lines) create moisture masses with different deuterium excess (d) values due to kinetic fractionation. Sea water lies at the 0,0 point on the graph. Evaporation of sea water at 100 percent humidity would produce vapor somewhere along the red dotted line pointing to 100 percent humidity, depending on the temperature. Higher temperatures would have lower fractionation factors and so the vapor would be closer to the sea water point. At lower humidity, kinetic fractionation affects evaporation, resulting in vapor plotting somewhere along the other red dotted lines, as shown for 85 percent and 50 percent humidity. The average global humidity at the sea surface is approximately 85 percent, so the GMWL intercepts the δ2H axis at a d value of 10. For a hypothetical region with evaporation occurring under 50 percent relative humidity conditions, d ~50 as indicated by the extrapolation of the dashed blue line to δ18O = 0. During condensation and precipitation, the d value remains similar for the vapor and the rain, even though rain or vapor samples will have very different δ2H and δ18O values along the blue dashed lines. This may not be the case for very low precipitation amounts and low humidity, for example in deserts (modified from Clark and Fritz, 1997).
The D-excess (d) is a proxy of the humidity of the source region, with lower humidity causing higher d values. An example of this is shown in Figure 21.
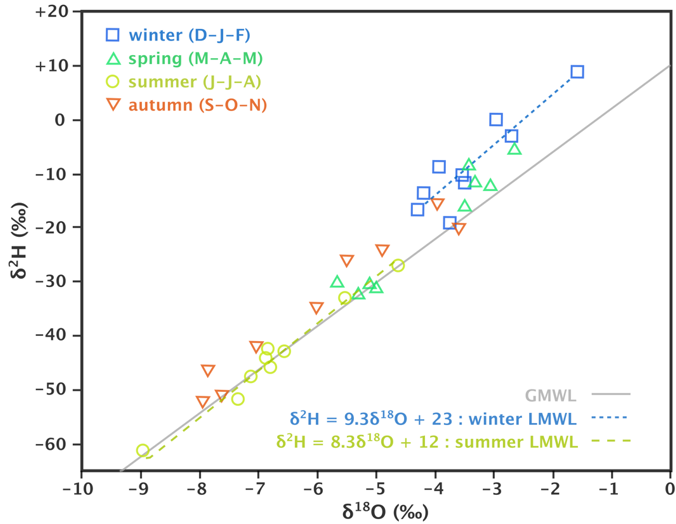
Figure 21 – Hydrogen and oxygen isotopes for precipitation on Okinawa Island, south of Japan, from April 2008 to April 2011. The distinct separation of the winter precipitation from the rest of the year is shown by calculating a MWL for the winter precipitation only. The winter data has a higher d-excess than the rest of the year, suggesting less humid conditions during evaporation in the moisture source region, which was postulated to be due to local evaporation around the island into a relatively dry continental air mass, compared to the other precipitation that was sourced in the tropical western Pacific (from Uemura et al., 2012).
