2 Isotopes and Isotopologues
An isotope is an atom of an element with a specific number of neutrons. An element is defined by the number of protons, for example hydrogen has 1 proton, helium has 2 and uranium has 92. Each of these elements, however, has a few possible isotopes – the isotopes of an element will all have the same number of protons, but a variable number of neutrons, which determine the isotope. For example, hydrogen has 1 proton, but with different numbers of neutrons: protium has 0 neutrons, deuterium has 1 neutron and tritium (radioactive) has 2 neutrons (Figure 3). As the mass of protons and neutrons is very similar, the mass of each of these hydrogen isotopes is approximately 1, 2 and 3 amus (atomic mass units), respectively. These are depicted as 1H, 2H and 3H. Hydrogen notation is slightly complicated by the alternative notation of H, D and T. Similarly, oxygen has 3 stable isotopes, 16O, 17O and 18O, all with 8 protons, but 8, 9 and 10 neutrons.

Figure 3 – The isotopes of hydrogen, including the two stable isotopes, protium and deuterium, and the radioactive isotope, tritium.
Isotopes can be stable, radioactive (decay to another element) or radiogenic (product of decay), or both radioactive and radiogenic, if they are part of a decay chain (Figure 4). In this book we will focus on only stable isotopes, which neither decrease nor increase in abundance, as they are not part of any radioactive decay chain. Note that radiogenic isotopes are “stable” from a physics definition, but as their abundance changes due to being daughters of radioactive isotopes, they are not called stable in a geochemical definition. As the abundance of stable isotopes on Earth is fixed, the ratios of their average global abundances are constant. For example, for oxygen, which occurs as 16O, 17O and 18O, as these are all stable, their relative abundances are 99.76 percent, 0.04 percent and 0.20 percent.
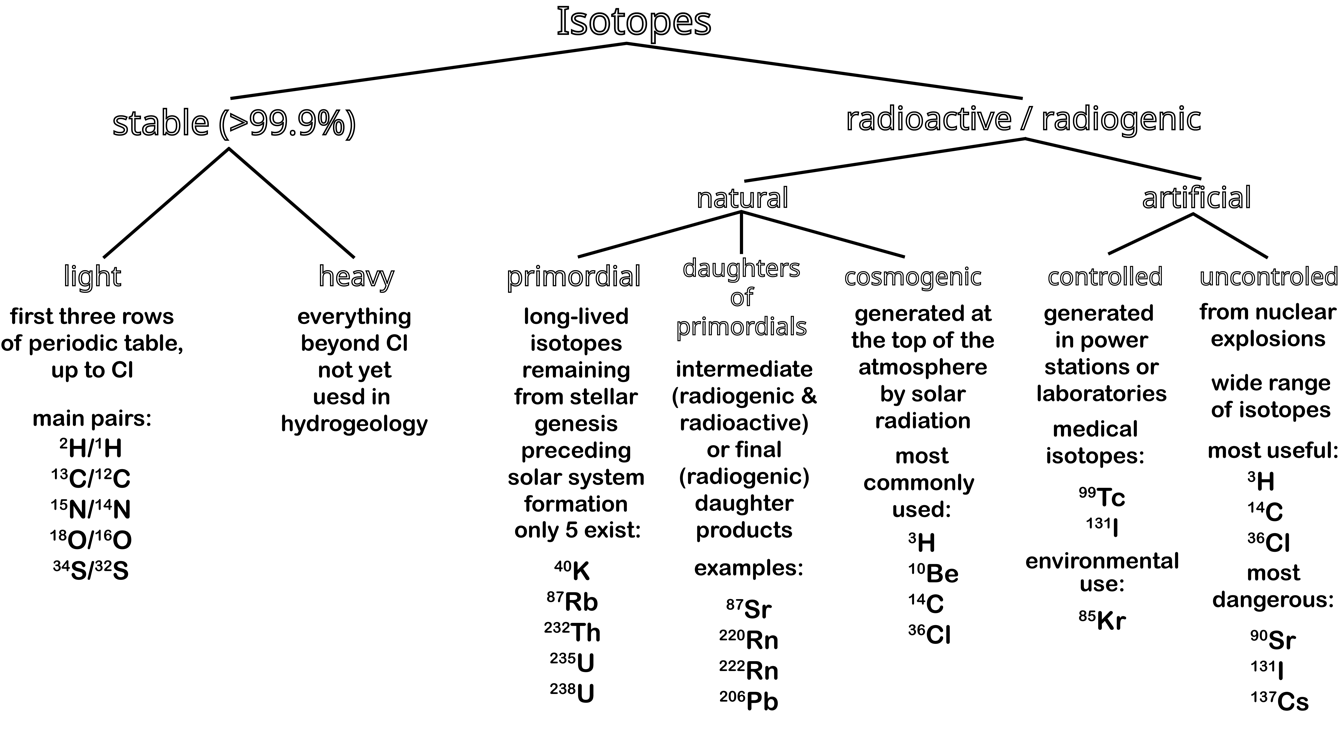
Figure 4 – Examples of the different types of isotopes, especially those of interest in hydrogeology. Geochemists define stable isotopes as those that are not only physically stable, but also neither increase (radiogenic) nor decrease (radioactive) in abundance.
Hydrogen is somewhat more complex, as 3H (tritium) is radioactive, with a half-life of 12.3 years. Protium and deuterium, however, are both stable, and on average make up 99.98 percent and 0.02 percent of hydrogen in natural waters, respectively. As with oxygen, you may notice that the lighter stable isotope is by far the most abundant. This is true for C and N and in all other light stable isotopes, but not always for the heavier stable isotopes. When displayed as a ratio, by convention the heavier isotope is always the numerator and the lighter isotope the denominator, for example 18O/16O or 17O/16O.
An isotopologue is an isotopic species of a molecule. For example, a water molecule can include 2 stable hydrogen isotopes, in 2 possible positions and 3 stable oxygen isotopes, giving 9 possible isotopologues (Table 1). If the possibilities with tritium (the radioactive isotope of hydrogen) were added, then the isotopologues increase to 18, but many of these are virtually non-existent. For example, the theoretical abundance of TD17O would be the theoretical abundances of each of the isotopes multiplied together, giving:
10–17 210–4 410–4 = 8×10–25 ≈ 10–24
for modern waters, which means it exists in extremely small quantities!
Table 1 – List of stable isotopologues for water and their theoretical abundances, as calculated from global average abundances of these stable isotopes (Emiliani, 1987). Isotopologues with tritium (3H, radioactive) have been excluded.
|
Isotopologue |
Mass |
Abundance (%) |
|
1H1H16O |
18 |
99.732 |
|
1H1H18O |
20 |
0.200 |
|
1H1H17O |
19 |
0.038 |
|
1H2H16O |
19 |
0.015 |
|
1H2H18O |
21 |
0.00003 |
|
1H2H17O |
20 |
0.0000057 |
|
2H2H16O |
20 |
0.0000022 |
|
2H2H18O |
22 |
0.0000000045 |
|
2H2H17O |
21 |
0.00000000086 |
A short note on jargon. Isotope scientists often refer to heavier or lighter isotope compositions as enriched or depleted, respectively. The convention here is that the words “in heavier isotopes” have been left out. So, an “enriched sample” actually means a sample that is enriched in heavier isotopes, and not a sample enriched in lighter isotopes. This slightly confusing jargon can be avoided by referring to samples with more of the heavier isotope (enriched) as having:
- heavier isotope compositions; and,
- more positive/higher delta values (delta, , will be explained below).
Similarly, for water with more of the lighter isotopes (depleted), we can say:
- lighter isotope compositions; and,
- more negative/lower delta values.
